Seminar 5 - Biomedical Device Development
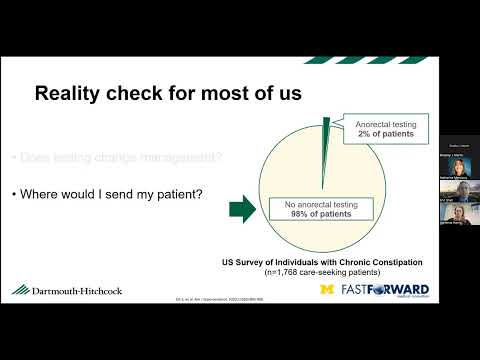
uh hi everyone and welcome I just want to introduce myself real quickly my name is Catherine mercica and I am the commercialization Education Program Coordinator at fast forward medical Innovation also known as ffmi and I wanted to welcome you to today's webinar which is number five in our six part Series so our last one will be next week uh the biomedical Innovation 101 series of webinars is provided by fast forward medical Innovation a unit of the University of Michigan medical schools office of research in partnership with the Department of internal medicine uh before we get started I have a few things that I want to cover including some technical housekeeping items uh if you have any questions that you'd like to ask today please type them into the Q a feature our uh staff will be using the chat function to answer questions and then share any additional links and resources and the presentation is being recorded and I will share out the recording and a pdf version of the sides to everyone along with an evaluation at the end of the program and with that I'm going to kick it over to Brad thanks Catherine yeah John Surplus is on a very well deserved vacation this week and so Catherine is filling in nicely for him so I appreciate everything John and Catherine do for the program uh again I for those of you that I don't know uh it seems like I know most of people on board here but I'm Brad Martin I'm the director for Fast Forward medical innovation and today's uh seminar is sort of a continuing series and this one's going to focus on biomedical device development and we're going to be discussing the processes for developing a biological uh device or development of a device some of the commercialization regulatory strategies that are involved uh Michigan has a long record of developing medical devices actually Therapeutics and medical devices are pretty close in terms of the numbers of patent disclosures every year by our faculty so there's a long history of device development here at Michigan and they run the gamut from everything from a very involved uh highly technical prosthetic robotic prosthetic uh or a um all the way down to a 3D printed very simple guide for helping implant subcutaneous implants and everything in between but there's some commonality amongst these things when we're talking about medical device development that's what we're going to be talking about today and so with that I've been introduce our two speakers for today um first you're going to hear from Dr Eric Shaw who is uh did his training here at Michigan but has since left and gone to Dartmouth but I'm happy to say it's going to be returning to the GI division uh here in uh in the early spring sometime um Dr Shah has been working with the GI division here at Michigan when he was here as a trainee to help develop a device to measure um just synchronous defecation but I think the lessons that he learns here and that he's going to be relaying to you are going to be applicable to a number of device development complications or technical issues that you may be facing so we're very happy to have Eric a coming back to Michigan as in general and also here and happy to have him here today to speak to us about his experiences in developing that device um then we also have our second speaker is going to be Adrian Harris who is the product development manager at a company here in town called intube and anyone who's involved in the medical device space here in southeast Michigan is usually pretty familiar with into being I would eventually say they are the preeminent company in terms of helping individuals and businesses develop medical devices and bring them to Market they have a wealth of uh of experience in developing devices the quality systems that are required to develop a system um and so we're very lucky to have Adrian here as well and I think she's going to be really kind of playing off of Eric's points and and his device element experience and talking about some of the common regulatory aspects or Quality Systems aspects to developing a medical device is that fair to say Adrian okay and so uh with that I'm going to turn it over to Eric and who I'm again very happy to have him here today and uh he can share his experiences in developing uh his red device rectal expulsion device Eric go ahead and thank you all right well uh thank you Brad for the kind introduction um and it's it's really good to be uh you know back in the fold with um ffmi and you know on this this call today with um Adrian and to share sort of what what the experience has been and um with you all so um we'll start off uh by kind of going through this project on what it looks like it's going to look very linear um as this goes I can assure you it uh has not been linear and um that is exactly what uh we'd love to discuss today uh just like science you know what is it ever linear um so we're going to be discussing the rectal expulsion device um to enable comprehensive constipation care for every gastroenterology practice to give some background here chronic constipation is more than hard and lumpy stools there's also straining sensation of incomplete evacuation Sensation that there's a blockage so a lot of patients can have diarrhea or so they think and yet they're constipated at the end of the day and constipation is an expensive problem so there's a culture of under testing uh with uh what gastroenterologists say are might be the most appropriate treatments there's a culture of overtesting so multiple repeated colonoscopies CAT scans Etc generates about 8.1 billion dollars in costs there's 700 000 emergency department visits um if there are any Ed docs on the call today uh can attest to this and 50 000 Hospital admissions related to a uh quote unquote flare of chronic constipation my goodness at least 3.4 billion of this uh could potentially be preventable if only we did the right thing so that right thing is defined over 10 years ago now uh by the guidelines from our American gastroenterological Association the AGA so we start with an interview and a physical examination with the patient we're going to consider a limited set of metabolic and tests structural evaluation if need be Baseline Labs the patient is going to receive fiber and or laxatives for a therapeutic trial and then according to the guidelines when there's an inadequate response meaning a gastroenterologist we do a test called an anorectal manometry in a balloon expulsion test this should theoretically be every patient that's seeing a specialty care provider the balloon expulsion test is conceptually simple so what happens is a balloon is inserted into the anorectum inflated with water the patient that moves over to a commode or a toilet and is asked to poop and if the patient can poop the balloon that is a normal result if the patient cannot poop the balloon or expel the balloon and it stays in then that is an abnormal result what we're looking for there are disorders of deprecation so there's dysenergic defecation we can call it this coordinated defecation but is it an anorectal issue is really the question we're trying to answer this is the related test and anal wrap the manometry so this is a sensor probe that's inserted into the rectum when the patient tries to poop the anal sphincter should relax which would be normal sometimes it doesn't and it actually strains squeezes and the pressure goes up and what follows then is that poop cannot come out it's stuck in there we call that dysenergia again there are multiple names for that if the test is normal and you do both of these tests together then you know I'm not seeing an evacuation disorder of the rectum I'm going to call it a Transit issue a motility issue I can treat that I have laxatives drugs different things in the Wheelhouse so this test has been useful if the testing is abnormal and suggests a defricatory disorder and those are patients where instead of prescribing the patient laxatives and drugs and making the stool loose I can send them to a physical therapist who can help improve coordination of the anorectal muscles and that treatment works but here's the reality check two percent of patients receive anorectal function testing among all individuals in the U.S with chronic constipation this was a study of 1700 patients across the U.S accounted for sex gender age geographic distribution um race ethnicity and only two percent of patients had undergone anorectal testing previously 98 had not what did their doctors say that weren't doing these tests you know this is the guidelines are not being followed 98 of the time they said does testing actually change management in my practice where would I send my patient if I offer this testing today why should I test every patient coming to my practice for chronic constipation this just doesn't make sense to me so we'll follow the patient perspective the patient starts off in Primary Care C is a gastroenterologist we do the best we can there might not be local anorectal testing available we're going to try the over-the-counter aisle the patient's going to come back we say eat more vegetables drink more water prescribe laxatives do a colonoscopy we're looking for that kink in the colon right prescription drugs and we get a prior authorization so we'll have a second colonoscopy I really want to find that kink in the colon surgery let's just get it out second opinions and repeated tasks phone calls frustration so a few patients are able to escape that they go to a tertiary Care Center they undergo anorectal function testing they have a second visit to sit down and discuss the results and that patient is then sent to a physical therapist so it's a pretty inefficient symptom and oh by the way there's a six to 12 month wait to get into a tertiary Care Center um so why not offer balloon expulsion testing today it's a pretty simple test so if we give this to gastroenterologists this is what we actually thought we might do originally we didn't know we needed to develop a device at the time uh when we gave this when we were going to give this to a gastroenterologist it would say um you know do I need to have a saline syringe and how much saline do I put in there um do I need to have a bedside commode in every Clinic room now I need to have additional staff and oh by the way I have 15 minute or 30 minute appointments I already have 20 other things to do um are my appointments going to be five minutes longer to account for that so I just didn't quite make much sense so we thought about this from a clinical workflow perspective and said you know wouldn't it be great if we had a bedside test where we could stream on this process make it easier and help get the patient to a physical therapist straight away um when there are two million patients coming with constipation of gastroenterologist each year so uh we developed red so it simplifies balloon expulsion testing enables point of care testing provides immediately actionable binary test results this is the device as it comes um and what happens is you unpackage it uh it uh has lubricant and is inserted into the rectum the end is uh popped off the balloon inflates and the patient is asked to poop um and that's it so we started prototyping this in 2017 we set out to do was build a cheap uh build it with uh materials that are uh biocompatible ideally and then build some non-investigational prototypes basically to turn it from something on a piece of paper into something that looks like it could work in the real world we did this by a systematic review of uh diagnostic test accuracy uh to try to identify how the existing gold standard devices work and how to optimize their design uh the best way we could to inform how we designed our device so what we found is that balloon expulsion test can be done in a seated position but it can also be done in the left lateral what is the left lateral position it's basically the patient on their side uh bright during and after a digital rectal exam so we can do that right in the office so which was an interesting finding um and uh and then we got the other parameters here uh that we um used a little bit later on so then we thought you know we have an opportunity now maybe not to build a water balloon uh to have patients expel as is traditionally done but instead what if we could mimic stool and have patients poop out poop and you know see what that shows um and uh fortuitous or lucky that we did at the time uh we didn't know but uh but this was uh something else we did um uh early on in the project so after that first year we spent two more years making devices performing some initial biocompatibility assessments um I'm going to defer to Adrian um on on uh discussing exactly what that is it's very important and um uh you know it goes to um why of these effective projects are most effective when um there's a team around them uh and uh there are a lot of things that as a clinical investigator I don't know and um and uh you know reaching out and through ffmi really helped us along that path um so I'd love to spend a bulk of our time um talking about that Eric can I interrupt you and ask one question real quick please so there was there was a device that was being used but this was not being used very often correct you said two percent of the time and so what were the key features of that device that existed previously that you did not like that you wanted to improve upon I'm trying to get to the point in which you decided we needed a bit we need to build something better what did you not like about the current one and how was yours that's an interesting question um you know what it comes down to for us was a willingness to let go of the guidelines um and a willingness to say hey maybe we don't know everything and we need to Pivot and I'll be honest that took a while um because you know I think in the clinical sphere you know there's a focus on the guidelines and then the guidelines are not being followed and therefore we have to help educate people on following the guidelines you know we found uh was we did this customer Discovery process where you have to go out and you're you go talk to a bunch of people yep and what you learn is there's a reason that people don't follow the guidelines and for us that reason was that having a saline syringe having water or saline or whatever you're going to use it just adds these steps and even if it's a few seconds long you know grabbing a medical assistant or a nurse somebody to help um it just eats up a little bit of time that makes this not fit into clinical workflow and so it was how do we take out all of those steps and you design something that may not meet today's scientific Perfection but can really help with that clinical workflow in a day-to-day World um so you know one of the things in the GI literature is interesting is that you know when patients poop uh the physiologic way to do it is when you're Seated on a toilet right no one poops on their left lateral position but the seated position doesn't make any sense um in a clinic uh because the patient would have to go down the hall to a bathroom uh which takes up time and it isn't Pleasant if you don't need to do that so you know with a balloon expulsion device you know so um so there are a lot of things that um that we learned in this process that really helped to inform not only the device but how we tested it um how we've been testing it um as we go so yeah that's it was customer Discovery I think yeah and I think the key point there is that for anybody who's on the call that's thinking about developing a medical device you need to be cognizant of the fact that it has to fit in the clinical workflow it has to be user friendly and not all clinical workflows are the same the way we do it in Michigan is not the way they may do it in the upper peninsula and so talking to people and ensuring that there's a broad customer base that wants this device to be put into the workflow is really important sorry to interrupt thank you very low Eric oh absolutely um so I'll quickly go through our trial data just to show you it's not so much that you know scientific side of um you know what this achieved it's you know how did we think about this from the standpoint of you know not necessarily commercialization but really getting something like this out there like what would be important uh for people to know at each stage so the first trial was really designed around making sure that what we built matched up in some sense to what the gold standard represents that was it um so we compared patients with chronic constipation patients who didn't have chronic constipation um and just wanted to see if the results lined up between what we built and a traditional balloon expulsion device the go no-go decision um you know if any of you have ever submitted or thought about an sbir Grant that's a key consideration that go no-go decision would be you know patients can't poop it out well we got to go back to the drawing board something's not right if Everybody Poops it out it's not going to give us much information either and we also don't want it to be painful so we ask patients if they if they could tolerate it um and um so uh so that's what our first trial was designed around the last couple years have been um three years actually been around uh the clinical use case so if we gave this to um practitioners what they're going to want to know is does the test change management okay um and that's it so what we did is we uh did two phases it was a feasibility phase what we were really trying to do is figure out what are the instructions for use of the device you know can we do a trial like this um and uh you know how do we write down those instructions how to use it how to interpret it in a way that um you know anybody so all patients who enrolled in this trial had chronic constipation um they were a general gastroenterology population which is important was not reflective of a tertiary care type um uh patient population it's already seen five other people um this is was intentionally um to enroll patients that would be meeting a gastroenterologist on the first pass um and uh so all patients underwent anorectal function testing all patients then went out to receive pelvic floor physical therapy in the community which was important so most of this was community-based pelvic floor Physical Therapy here's another schematic showing sort of how we designed this trial uh so patience we just made sure they failed the typical fiber or Miralax for a couple weeks at least and then they came back all patients got tested all paid patients went to see a pelvic floor physical therapist and we did pre and post treatment outcomes and uh what we came up with is you insert red into the rectum remove the cap and then the patient would sit in the left lateral position for a couple minutes I might go chart and then just see see if the balloon is still there two minutes later and then come up with a a game plan with the patient based on that in the trial we added a seated position to try to see if that also would help get data the treatment protocol and this is this was interesting so we went out to talk to pelvic floor physical therapists and you know part of the customer Discovery is we just asked them you know what do you do for a living tell us what you've done for the last 20 years um is the treatment going to be reflective of What patients would actually receive for to say million patients with constipation in um you know anywhere um so we were the referrals were balanced across uh sites uh the treatment was part of clinical care um for patients um the treatment did include this uh called biofeedback so it works on the anorectal muscles as a primary treatment modality um the protocol was basically followed what they already do which ended up being as the same as a simplified protocol that uh that is a bit easier for patients to um uh to navigate the traditional protocol calls for several visits um to a physical therapist a physical therapist here you know much like anywhere said you know there's there's a limit to What patients will will take it's hard to do pt five days a week um you know without affecting you know other aspects of life and work so uh we aim for about three visits with each physical therapist um the point was that the physical therapist designs our treatment protocol completely and it basically followed what they already do um so we designed this like a trial so we had a primary outcome uh to achieve a minimal clinically important difference in a uh outcome measure called pacsim that measures Global constipation Improvement so we could stratify patients or responders or non-responders we use the same sort of measure for health related quality of life responder non-responder and there is an FDA on point for drug trials around whether patients are able to have a better stool frequency and form that doesn't measure the quality aspects of defecation that go into the primary endpoint but we adapted the drug trial outcome just to get that information at this stage nonetheless so the analysis was abnormal versus normal test result responder versus non-responder and we could calculate an area under the Curve um and um we use this it's called a yowden index we could use this uh statistical method to figure out how you would optimize how to interpret a test like this uh to inform uh what you would do in practice so of the 60 patients uh we had 52 that were included in Our intention to treat analysis meaning that they attended at least one physical therapy appointment uh so we had 88 percent numbers work out too 88 percent adherence um and what's interesting is that at Baseline a fifth of the patients we're pooping at least three complete spontaneous bowel movements per week they were pooping but they had sensation they couldn't get the stool out they were seeing a gastroenterologist for constipation so just underlines the constipation doesn't just mean hard lumpy stools uh there's one adverse event so anal pain with the suspected anal fissure um that pain resolved immediately was replicated when the patient underwent interactive manometry and then also with pelvic floor Physical Therapy um so anal fissure um so and that is expected uh in this um in this clinical disease entity to no serious Adverse Events so Baseline demographics here we're similar to what we see in chronic constipation trials it was reflective of uh what general gastroenterologists should expect to see we did get Baseline interminometry testing as well along with that and what we found is that the test was able to predict clinical outcome are areas under the curve or between the 0.6 to 0.7 range which was
intentional based on our power analysis we were not aiming to identify a test that could 100 percent uh predict uh that a certain group would have a 100 percent rate of success with pelvic floor Physical Therapy compared to a zero um so because we thought that the responder rates and Placebo and all of that might be smaller the resulting AUC out of this was expected between 0.6.7 uh so I did meet on global symptom response um health related quality of life and then also this bowel symptom response um in the left lateral position it wasn't power to do that uh so it trended towards um towards um uh demonstrating that might work in seated position but we can't claim that as it wasn't powered to um to do that so in the seated position all right how would we Define a positive test who am I going to send a PT so if we retain the device for at least 13 seconds okay in a seated position I would expect a quarter of the patients I see uh to test positive then I would refer that patient to a physical therapist um you know if this device were approved and we'll talk about that sensitivity and specificity are here higher specificity interestingly so an abnormal result would would mean that I could tell a patient I think you're going to have a 71 chance of responding to pelvic floor Physical Therapy versus a normal result says 28.9 percent so there's a p-value there statistically significant in customer Discovery we learned that gastroenterologists like the 28.9 percent they're going to refer that patient of pelvic floor Physical Therapy too okay so yes you can predict who's going to improve with pelvic floor Physical Therapy but I'm still going to send most patients the reason is that prescription drugs and other treatments laxatives have a 30 percent rate of efficacy anyway so um the Paradigm works but it wouldn't change practice so the left lateral position was the most interesting so here uh if the device falls out within five seconds or they retain it for more than two minutes then three-fourths of the patients will test positive so most of those patients I would send a pelvic floor physical therapy just to try it so we have a high sensitivity test what's interesting is that with a normal result so if the device um takes a little bit more than five seconds um to fall out but isn't retained for at least two minutes those patients don't respond to pelvic floor physical therapy so I can help that patient not go to get internal rectal physical therapy and try a different treatment who would otherwise otherwise be willing to go so that was that fits with uh what the use case would be in a general GI practice and and how they would like attest to to perform and um so that was very interesting so you know just just kind of spark the discussion here um you know one thing we learned does the idea we have solve a problem that people actually care about okay we think yes right the answer is probably no so you know what we developed originally had uh looks nothing like this and you know I don't think we knew what we were going to develop and that was entirely the point that um it took going out and talking to people who aren't quote unquote experts um but you know there actually are experts um and so that reflects most of clinical medicine you know so learning that um what the day-to-day life and challenges are like um even though we think we know it um it was really helpful um you know and even then is it sufficient for investors is there a business around it um would the FDA care um you know or would it not be good enough so there's this three question approach to commercialization so I think John cervas um and um Jonathan Fay um you know led a course several years ago um and so you know this was from what Jonathan Faye had um presented you know is there a value prop is there a viable commercialization plan is there a return on investment okay that three question approach drilled over and over and over and rinsing and repeat until you find something that might work um you know I I think this would have been a fun science project if not for um you know meeting Adrian and working with into being you know um and um you know being willing to Pivot um and you know that's where you know we might have a great idea um the engineers know if uh if that's actually something that's going to work um I think so the Rapid Care pathway for constipation as we would potentially frame it now uh would be that a patient would come to a gastroologist for constipation or to an Open Access unit for a diagnostic colonoscopy for constipation which by the way is not reimbursed for that indication chronic constipation does not cause um colon cancer change in bowel habits might but chronic constipation does not the first time you get a colonoscopy fine the second third fourth time I have questions okay so the patient could be seen in the office undergo red the normal test optimize the bowel regimen abnormal test we engage this evacuation disorder paradigm so you know what I think was really cool so you know my mentor on this is um Dr Bill Che um he's the chief of GI now um and you know the way the way things are going in medicine you know how do we help patients get to the right treatment faster um how do we create these clinical care Pathways to do that uh you know within these big black boxes um you know that we have in medicine um and then how do we leverage that clinical infrastructure that is wonderful with new medical technology to get there and so that's sort of you know the things that um uh that fall in line with this project and with others um that you know I'm really looking forward to in um in uh coming back and it's it's going to be a really exciting time so uh so this is you know probably up two percent of the ecosystem in uh the university and in Ann Arbor um there are a lot of uh great people and resources um you know across the university and around Ann Arbor so into being um there's a uh robust um Venture Capital presence um there's spark um there's incubation for incubator um and then ffmi and Innovation Partnerships can is um uh you know I I felt like has been a a hubbed uh learn and uh sort of navigate uh all of this because uh there's a lot out there and uh which is really cool so uh here's our contact information um and uh so you have my email um we have Adrian's email here um and uh yeah we have a lot of time left this is great well thanks Eric I very much appreciate it and um the your I appreciate your kind comments too about the the course and the value that you found in that course and just for everyone's information that course is still being run by our office it's called the fast-paced program and it's very much focused on customer Discovery customer segmentation figuring out your value proposition and coming up with the go no go decision on your your device idea so we do run that course still and it's a very impactful course for a lot of different people Catherine just put the link in the chat feature there so that's uh another version will be coming up this spring another cohort we've run that almost 18 or 19 times so far so um Adrian I didn't know if you had any comments on just sort of the not necessarily related to this project necessarily but you worked with a lot of U of M innovators and a lot of medical devices just general comments on device development regulatory considerations Etc sure make sure I unmuted myself this time I'm going to use this project as an example because you know Dr Shaw has laid out this beautiful development and Science and how to how to get a device to show value um it's had a lot of really unique regulatory challenges for something that seems so simple uh very low risk all that put together um it's had a unique path through the FDA so far um there's also been unique opportunities for this device in that as an example it's considered a non-significant risk device so it allowed the clinicians to get their device to a clinical trial um on a speedier pathway than if it fell into not into significant risk um it's a non-sterile device you don't have to prove sterility before it touches patients all that great stuff so we were able to get science moved forward um while mucking through uh the regulatory side with the usfda so a little bit on that the first time we interacted with the FDA was with a 513g and that was to get buy-in or feedback on what the actual product code and regulation number this device should fall under and looking at what's out there a lot of the devices that do balloon expulsion testing were kind of shuffled in under the bigger anal rectal manometry systems uh we looked at this and went you know this doesn't have a big electromechanical regular system to go with it it's just a balloon there's a product code and a regulation for balloons that get put in people for Diagnostics we want to pitch the FDA that this should really fall under that uh Dr Shaw reminded me that this 513g did go in during the government shutdown a few years back I don't know how much that had a role and then don't worry after that we rolled into kovid which also drastically changed interactions with the agency they came back and said no we don't believe that you're just a diagnostic balloon and I'm going to read this just a little bit now that you've seen the device and you saw what it does and how it works they said here's here's the regulation you fall under a motility monitoring system that is used to measure activity and pressure in the stomach or esophagus by means with a probe and transducers it's introduced through the mouth it goes in the gastrointestinal tract it includes signal conditioning amplifying and recording equipment it could have certain accessories such as pressure transducers amplifiers and external recorders your class 2 device go through the 510k process did you see any of that on the device no but because the predicate devices that do balloon expulsion testing came with the systems that do all this other stuff and according to the 510k process and the FDA foundations that's the category they wanted to put us in okay we can work with that the follow-on to that was a pre-sub to say all right you put us in this classification we've worked hard to find a device that could be a predicate however we want to check with you that all these things that were going to come into your predicate system with um you know the point of 510k is the show substantial equivalence to something that's already out there all these other systems have big electromechanical equipment they do have pressure transducers they do signal processing they do sensing they do recording we don't do ours the unique challenge in that is the majority of the devices that are used on the market right now were cleared uh through the agency 30 plus years ago which means you don't have access to the 510ks to look at even what they did clear it was a challenge and the FDA would like you to use a device it's a predicate that is within 10 years old at this point they don't like you bringing something on to predicate to something 30 years old we found a device that was recently cleared um in the late teens 20 teens that had a balloon portion that did not hook up to all the transducers did not hook up to the electronic system and we went to the FDA and said would this work for a predicate if we just predicate off of this portion of the system and gave him all the justification and whatnot in that and he said yes it's like fantastic we have something to go on um what was interesting though is in that particular device they don't actually have an indication for balloon expulsion testing it does everything else it looks exactly the same it falls under the regulatory requirements and product codes and regulations but doesn't have the same indication so as another challenge in how we were going to present the device to the FDA when it does go through the 510k process and then that's where we'll get to tie in all this clinical data that the clinicians have been able to to gather in support of it which is what the FDA asked for in the in the pre-sub okay you can show us all this stuff with bench testing that it's very similar to the predicate but we need to see some clinical data based on the differences that it does do the same thing and this is all in the background well you know actually trying to develop the device it's figuring out how you're eventually going to get that device to commercialization through the regulatory challenges of the United States FDA and that's where my work has come in to help more so even on the development side but we did start with the development of the device at the beginning to my anecdote there is uh this is the first time I've ever had to do Force testing on what is considered healthy stool that was a unique process in and of itself to determine what actually healthy stool behaves like so it's been a fun fun ride all the way through you are on mute I do that all the time maybe um maybe just for the benefit of those who are perhaps very naive to the the device approval process could you just sort of tell them the difference between a de novo pathway and 510k pathway you talk about predicate devices people understand what you're talking about absolutely uh one step above that the FDA has three classifications for devices class one class two and class three class one is the lowest risk it means you do not need the FDA to review anything about it before it goes to Market make sure you follow the qsr which is uh 21 CFR 820 you meet all the requirements within that regulation and you may go to market with your device if you cause problems the FDA is still going to come look at you they can still inspect you at any point but they don't have to pre authorize your community your commercialization I'm using authorize because now we're going to get into the details the 510k is for Class 2 devices the 510k is based on there being another device out there that is very similar to yours that you can use as a predicate and what you're doing with the 510k is you are demonstrating substantial equivalence not the same but they are substantially equivalent to something that's already on the market and if that's already been proven safe to be out on the market then if you can show enough similarity to your device then you can be safe 510k another alternative name for that is a pre-market notification and if the FDA grants it you get clearance so not approval per se and you don't get clearance or approval for a class one so you get your your 510k cleared and then you are cleared to go to market class three highest risk devices is called a PMA or a pre-market approval this is when you would actually use the approval word device I'm going to skip over that one because it's outside of the scope de novo is very interesting in that it is for what the FDA considers automatic class 3 designation but where you go in and then they review it to the point where maybe you don't you aren't actually a class three and where this comes in very um useful and kind of cornerstones with the 510k process let's say you have a device that from a risk-based perspective isn't a class three it's not a class one it really should be a class two but there's no predicate out there if you don't have a predicate that you can take to the FDA of to go through the 510k process then you become a de novo and a de novo when you're done you get a classification and a regulation number maybe you already fit into a regulation but you don't have a classification yet for a device once that device is granted the de novo if it is granted a donovo for a class 2 all their products including products that you're you develop afterwards that are very similar can use your device in the 510k process but it needs to have that initial Foundation device to say it's allowed on the market as a class too the unique difference between a de novo and a 510k for a device that's ultimately going to be identified as a Class 2 is De Nobles require clinical data this can be a major hurdle for a system or a device that um it doesn't seem like it should have that higher risk doesn't seem like it should need clinical data because it went through a 510k process you would not need clinical data but that is the big uh gap between going through a 510k process through de Noble here if the FDA came back after our pre-sub and said no you know what all these other products are too old and you don't match them enough you need to go into Novo not a huge punch in the gut because the team was already getting clinical data but if you weren't planning on that initially that's a huge cost that needs to be taken into consideration for your development process great thank you any other questions from the group here in our final 10 minutes for either uh Adrian or Eric just go ahead and take yourself off mute and don't be afraid to chime in and ask a question Medina I know you had a question down here in the Q a I don't know if you're fanatically answered yet it must have been going once going twice it's a new oh a couple more questions uh from Charles Schuler probably to Eric how did you find the commercialization how did you find the commercialization process yeah it's um interesting uh hard but fun and it's it's a different sort of set of questions than the um usual uh sort of you know scientific inquiry but it's it's very similar um you know it's it involves really answering that question do people care um enough to care with money um and um you know and that goes to impact you know does your you know paper your project that you're publishing you know is it generalizable is it novel is it rigorous citable you know all that so so it actually is quite similar in that sense um you know the key is who's the audience the audience is not going to be key opinion leaders in in your field per se um because you know if you're you know on this call and thinking about commercializing an idea you really want to um uh develop that idea around um so-called non-experts but if you flip that the non-experts are experts and their challenges are very different and so that's really where I think the uh commercialization process comes in is learning how to think about you know what that means um day to day um which was um you know which which was really cool it's a it's a it's a process that's that's what I think the fast-paced course was um you know particularly helpful for and Eric wow we've got you here um one of the participants Medina sokolov is asking you about your career stage when you started doing this work and did that make a difference in terms of what you were able to achieve or not achieve um yeah that's that's a good question so so I started this when I was a uh uh clinical fellow in GI um I in 2015 and so like before this work was a year of learning about the commercialization process through ffmi and there were a series of Grants there was a GI Innovation fund at the time and they had two tiers of Grants and um you know I think that in learning through the ffmi courses um really taught us gave us the skills to learn how to be successful on the grant side for that um you know and since then we've um you know had some professional Society funding for this as well um you know I I will say there is federal funding opportunity for this um type of work either in the sbir programs um and then um you know you could use traditional Pathways for it um yeah it you know it it becomes tricky if you're at the stage where you know this is a company per se um but you know that's where there are mechanisms out there um you know at different stages um to do this type of work and you know I think I think ffmi is good a great resource for that as well um because they'll know everybody who has you know been not successful in certain ones which can help and successful in other ways um as well so and that kind of gets into another question we had here from someone else asking about the funding available through either fast forward or into being and um a number of years ago we used to administer a program for the GI division which is made possible by a grateful patient and a gift that she made and that's how air came about working in this space but there are a variety of funding resources um that exist on campus that we can point you to some are administered by our office some are through the Coulter Foundation or through the tech transfer office but we can certainly put you in the right direction um Eric's comment about sbirs I often hesitate to talk to people about sbirs because I think that forming a company and going through the process of forming a company for the sake of getting funding it's sort of a batteries in the form of a company let's form a company when it makes sense to form a company going after SBI dollars um is not the best reason to form a company there are other resources on campus that allow you to de-risk a technology or a device or build a prototype or do some initial clinical testing before you get to that stage of forming a company and that's what exactly what those translational funds that are available to you through the university are made for they're non-dilutive they're not they're just research dollars that are for you to develop a clinical um a clinically viable product um and one of the other things I want to mention too is that we can do an awfully good job of doing some matchmaking between clinicians who have identified a problem and Engineers who can build it don't think as if you have to be a a jack of all trades as well as the clinician to build a medical device we can do matchmaking between colleagues that are at the College of Engineering who are have a lot of know-how and Technology but they're just clamoring for a clinical indication to apply it towards um and there's also people uh in the local area like into being an Adrian who can help as well so if you have any questions about funding just know that there's a lot available um and come to our office and we can point you in the right direction many of the many departments have internal funds there's the Franklin Innovation fund there's Amtrak there's a new program that's being put out by Steve Kunkel in the office of research called bold science which we're trying to fund some outside the box ideas um so a lot of it is available to answer on intervene's part we don't provide funding opportunities specifically but we have collaborated with a lot of researchers researchers at U of M to help develop some of that writing for imtrack Kickstart other types of Brands whatever you're going for to help lay out a development plan that is made by people that develop medical devices again we don't expect a GI doctor or a cardiovascular surgeon to understand the whole medical device development process you have a great idea and what we do is we can come in and help lay out how to get that idea to the next step how to get it to the last step and then also work with the team on that throughout the course so that is one thing we have worked in it we've worked with uh on the red device in that regard to helping get funding for it as it's moved through the process and the one that I can say is with absolute certainty is that in my 10 years of reviewing these translational research applications for funding you can very much tell somebody who has spoken with into being or has been through an educational program and understands the processes the language changes their understanding of the uh of the market and the path of virtualization uh it was much more solid and so taking a course is usually your first step talking to somebody like Adrian on YouTube Beans use your first step and that really enhances your chances to get funding Downstream so and there's the link for interviewing thank you Catherine any other questions here we're getting to the end of the hour here and I want to make be sure to respect everyone's time um are there any other questions Catherine can people join in uh by Audio or is it just through text I think it's through text but let me or chat let me think maybe that is not the case I think I can individually go and let people talk so um yeah you should be able to I think raise your hand if you want to one last thought thoughts comments or questions otherwise we will let you go all right Adrian and Eric you have our Eternal gratitude thank you so much for um taking the time out of your busy schedules to present today um again if our faculty have any questions they can either contact us or the speakers directly at the links that were provided and uh hope you all have a great day and thank you again to our speakers okay thank you bye-bye
2023-02-20 23:08