PATIENT SAFETY MOVEMENT MIDYEAR MEETING 2016 - INOVA HEALTH SYSTEM KEYNOTE
everybody could please take your seats we're gonna get started after dr. Jeff Shirin speaks we did forget one of our regional chairs dr. Xavier Davila which we're gonna ask him to come up and give us an update on from Mexico but we'll do it after we're gonna do it right after dr. Jeff Shirin just give a few more
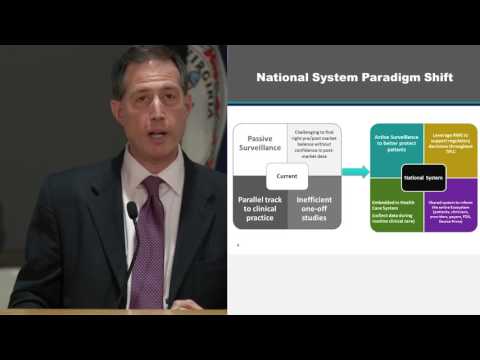
minutes please I'm really excited I'm proud to have dr. Shuren join us today as head of CDRH for the fda jeff has done a fantastic job of having this huge tanker and moving it in the right direction where there's a creation of better balance between minimizing risks but also not not slowing down innovation from reaching patients which has its own risk we've tried to have Jeff join us in California for a few years we finally had to bring the mountain to Jeff so we're really glad we're here so without further ado dr. Jeffrey Shirin please thank you thank you Joe thanks all of you appreciate the opportunity to come talk and and to bring the mountain if you will over here well I get the opportunity to talk about something that's near and dear to my heart it's establishing a national evaluation system for health technology we put health with a small H and we say health rather than medical devices because her interest is in technologies and information that can be gathered from technologies beyond what may be considered as traditional medical devices and I'll talk about that and from the FDA perspective patient safety and jus Joe alluded to this is more than simply assuring that we don't have unsafe devices out on the market and not just about identifying safety problems more quickly but it is facilitating the development of safer more effective technologies and that's what I'll talk about and how this approach can help achieve that so our vision for the Center and for the medical device program is that patients in the US have access to high quality safe and effective medical devices of public health importance first in the world this is not about a competition between countries it's simply a recognition that we want devices to be safe we want them to benefit patients but if they are they're of no value to patients unless they have timely access and first in the world is simply a good metric for that and we face in a challenge here in the US we have a fabulous standard for patient safety particularly as applies to high-risk and innovative lower risk devices and that is reasonable assurance of safety and effectiveness why it's one of the highest standards regulatory standards in the world because for those technologies you have to demonstrate a benefit usually with clinical studies most of the rest of the world looks at safety and performance the challenge though is you will then need more evidence to get that product high risk innovative lower risk devices on the market in the US and that can create a disincentive for innovative's to bring their technologies here first if at all so over the past few years we have been focusing on trying to reduce the time and cost of devices coming to the market in the US and staying on the market but not undermining that standard of reasonable assurance of safety and effectiveness and that to us is the sweet spot one of the underpinnings that we have pursued is what we call flexible regulatory paradigms rather than the traditional approach of you take a technology and you put in a one-size-fits-all pathway we are designing regulatory pathways around the technology around what the evidentiary needs are the innovation cycles and patient access and we've also been putting in place far more flexible policies on benefit and risk not just explicitly stating the factors in how we consider those factors in assessing the benefits in orissa to technology but the societal trade-offs about when that technology comes to market specifically how much uncertainty do you accept about benefits and risks before product enters the u.s. marketplace understanding that
you may want to accept more uncertainty when we have a technology treating a life-threatening condition and that may mean earlier earlier market entry for that product and that's a hallmark of something up here called our expedited access pathway it's a pathway for breakthrough technologies the last piece here all the way on the right it's about the science of patient input so when you think about benefits and risks you always have to put a value on it question is whose value and at the FDA traditionally it would be the Preferences of the reviewer where as we all know technologies are used on or in patients and therefore we'd rather be relying on the trade offs patients want to make but how do you assess those preferences in a scientific manner such that you can rely on them and that's the science of patient input we've already been doing Studies on patient preferences and starting to incorporate that into our decision-making but ultimately the real key to success in getting product on the market staying on the market adoption and reimbursement is evidence it's the science behind it and there been a number of developments over the past several years that helped put us in a very different place of course the Information Age has resulted in rapidly expanding computer power it's estimated that in the next thirty years the power of a single computer will exceed that of the combined brainpower of the entire human race that's startling and secondly we all know about increasingly more data out there in fact it's estimated that 90% of the world's data was just created in the past two years and that creates wonderful opportunities but unfortunately today we deal great inefficiency is in our healthcare system as you well know there's data generated every single day as a part of routine clinical practice but we can't make good use of it because systems don't talk to one another that data may be of poor quality may not be complete different definitions are used by difference data collection tools even though we may be talking about the same kind of data points and there are inadequate methodologies and in addition today data is wrapped up in silos we are competing around the use of data rather than freeing up that data and competing around the technologies that are developed and understood through that data so what does the world look like today in terms of our assessment of medical devices well we rely very heavily on one-off clinical studies a traditional clinical study is incredibly expensive to conduct particularly here in the US all the administrative burdens to go ahead and set that up and it's got terrible limitations as you well know because often the patients who are being assessed in a study which needs to be so pristine to tease out benefits in that smaller population may not be truly reflective of the patients who are going to use that technology once it's out on the marketplace meaning we don't have necessarily a great understanding of the benefit risk profile of that device moreover to truly assess and understand the full benefit risk profile you have to look at a lot of patients and you can't do that prior to marketing would be so prohibitive nothing would ever get on a marketplace and when it's out there oftentimes to identify a new problem or a greater frequency of a known risk we rely on passive surveillance we rely on an individual usually a practitioner to identify there was a problem identified there's an association between the use of a device and that problem and then report it to the FDA or the manufacturer as a result things may be missed or a lot of patients are exposed to an to a device and experience an adverse event that maybe could be avoided before it's actually detected and clinical research oops sorry and clinical research tends to be separate from clinical practice and finally because we have so much challenges in gathering data in the post market setting in clinical trials because once a device is on the market patients generally lack an incentive to enroll in clinical studies it's very hard to find the right place in drawing the line of what evidence we need to go to market versus what we may need or be able to push into the post market setting that we know we can accept more uncertainty in some cases but its premise on the fact that we'll resolve those uncertainties quickly after the product is approved so where do we go well this goes back to how do we better leverage that data being generated in routine clinical practice whether it's in healthcare facilities or in the home what we call real-world data to then generate real-world evidence because we think the values here are the following instead of looking at passive surveillance to identify problems going and taking advantage of larger data sets that are out there but then applying more sophisticated analytical tools ones that we already have developed and start to make those associations between adverse effects and the technologies being used if that data can be better used then we know we can make shifts in the data we collect pre market vs. poem's market because the data is already being collected it's not about necessarily enrolling people in a new clinical trial and moving clinical research to be embedded into the workflow of routine clinical practice that ultimately is the big solution so what we have put on the table is establishing this national evaluation system for health technology that is leveraging that real world data both to facilitate products coming to market and putting in place a safety net once those products are being used out in clinical practice and in 2012 we had proposed this system the idea is how we leverage data that's already there in device registries electronic health records patient claims forms and incorporate within that information what we call a unique device identifier today there are so many thousands of technologies on the market and often time those records may not be specific particular electronic health records payer claims forms on what device was actually used so we have developed a system under which medical devices in their labeling sometimes on the product have to have a unique code and now about incorporating that into electronic health information so you can finally link the use of a device with the patient's experience with that device and the purpose here is how we develop a better understanding of the real benefit risk profile of the technology and feed that back to providers to patients and to developers to more quickly identify safety problems so we can address them more quickly to reduce burdens on the time and cost of evidence generation coming to market not sacrificing the robustness of the data but intelligently reducing time and costs I'll explain why in a minute and then ultimately facilitating then the approval or clearance of safe and effective devices for patients for providers for payers talking about better information for making the kinds of decisions that they need to make and on the industry side reducing the time and cost on evidence generation reducing some of the regulatory burdens they currently face but not sacrificing safety and effectiveness ultimately we think having more robust data in the right time and in the right way to inform better decision making and we have already been leveraging this kind of data in some of our decisions and I'll give you some examples in just a moment so what are the key features of nest what are we talking about well this is essentially it it's to drive down the time and cost and increase the value and use of real-world data to meet the needs of the medical device ecosystem stakeholders through a market driven collective buying power approach using a neural network model can everyone say that again three times fast but let me walk this through because this is much more of a market-based model it's not a traditional approach that we have used at the FDA before so at its heart is an independent coordinating Center one of the challenges we have today in the use of data is the marketplace for data so to drive down the cost of the medical device when you buy it well the groups band together healthcare facilities can band together that collective buying power drives down cost but we think about the time and cost and use of data we don't necessarily have that marketplace for the data for our understanding of medical technologies so nest is about bringing together represent representatives from the ecosystem and establish that kind of buying power starts with an independent coordinating Center who is now focused on using that buying power to then force or if you will encourage standardization in terms of the core data elements that are collected data quality use of common definitions linkages between data sources linking up registries and electronic health records and payer claims the development of advanced analytics and creating the data use agreements with data owners we're not talking about creating a new system of data it is about coordination governance and standardization in the use of data that is collected and still retained by the data holders so that's a federated system the oversight honor the governance structure is comprised of representatives of key stakeholder groups in the ecosystem so that is its industry its providers its patients its payers its government so FDA doesn't own the system we don't run the system it is of by and for the ecosystem we have a seat at the table very different approach than we've talked about previously and the last piece then is the business model so we have been trying to work with the device industry with Congress about getting seed funding to get that coordinating Center up and running and keeping it operating and some basic funding to invest in setting up core structures but then the ultimate use of the data comes from those who are interested in accessing the system than paying for its use but the cost down because of the collective buying power and the success then on nest is because the return on investment for going through nest as opposed to setting up a traditional clinical trial is there's a greater return on investment I mentioned the neural network model so what are we talking about the traditional model today is you want to do a clinical study you may go through a coordinating Center you establish relationships with clinical trial sites you conduct your study wildly expensive lots of individual contracts going through separate IRB s it takes a long time and then when over and done with maybe you can reuse it but still very challenging the neural network is essentially a network of sub networks relationships in this case between data sources that are then if you will activated depending upon the question that you're answering so I'm a neurologist this is why I think about neural networks so much like the brain works this is very similar so let me give you an example today the FDA has a system we call Sentinel it is a sub network of owners a pair claims data that are then linked together and the FDA when the FDA has a question they may pose the question to that body of data owners and they have a coordinating Center and they do an analysis to answer that question that's claims data the government established P Cornette which essentially is a sub network of electronic health records it too has a coordinating Center and there are studies that can be conducted out of P coordinate but the governance structures are very different they have different interests nest is about a governance structure representing the interests of the medical device ecosystem and establishing those linkages between systems like Sentinel peak or net and medical device registries linkages that we have already been in the process of setting up that's the neural network model and depending upon the question your aunt's asking or the study you want to conduct you may be looking at leveraging registries electronic records a combination of the two payer claims again it depends but you've established the linkages you've established the relationships you have common in court datasets definitions and therefore the cost to use that system is dramatically less than what otherwise occurs in a traditional study in setting up the system we established two multi stakeholder groups so there was representation from key stakeholder groups I mentioned previously one was a planning board who put out a report last year on the steps to take additional steps to take to set up the system they have also established the criteria for the coordinating center and we have put out a call for nominations for a coordinating Center we have received those nominations and we are currently vetting them and if the funding arises for us to continue the coordinating center for a few years the agency is in the position to provide the seed funding to get it off the ground which we would do the second group was a medical device registry task force that focused more specifically on unique issues with device registries identified where there were needs for additional device registries and talked about something called a coordinated registry Network essentially strategic alliances between more like situated registries a sub network if you will recognizing that today with all the data quality issues one data source may not be fit for purpose but a linkage between two or more may be and therefore in setting up the system we don't have to have perfection instead those linkages can make do for inefficiency as we currently have you are in effect building the airplane while you're flying it that's what we have been doing in fact we've set up these kinds of coordinated registry networks already for example we have the international consortium of orthopedic registries so over a dozen countries participate and now linked their data together on orthopedic implants we as an agency have been making investments for the past five years we've set up that unique Device Identifier system as of now all high-risk devices have them in a few weeks essentially all moderate-risk devices will and in the next two years the low-risk devices will have them all either in their labeling or on the product itself we have complete are engaged in over fifty projects already that are starting to demonstrate return on investment creating new data sources new analytical methods and demonstrating the concept of use of real-world evidence more generally and we've already invested over thirty million dollars in getting this up and off the ground as I mentioned we've been using real-world data already so the American the Society of thoracic surgeons the American College of Cardiology set up the transcatheter valve therapy registry a few years ago in coordination with us with the Medicare program and with some of the companies who were developing transcatheter aortic valve replacement devices and we those two societies and one of the companies were about to do a clinical trial to look at alternative access modes for that device we looked at the data in the registry and felt the answer was already there we contacted the societies in the company and said cease and desist there's no reason to come to us with the clinical trial protocol in fact instead asked us to expand the labeling indication and in a course of three weeks we did it as opposed to spending a small fortune and over a year to go through a traditional clinical trial we have been using registry data as a control arm in pivotal clinical trials what does that mean so for devices like this instead of enrolling patients into two arms you're enrolling them just into one arm again big decrease in time and cost not sacrificing the quality of the data that we're getting we've been using database information to demonstrate the clinical validity of diagnostic tests in fact we've done that in a case of next-generation sequencing for cystic fibrosis so again didn't need to do a new clinical study this data was in a database that Hopkins was maintaining and then for post market studies that we may require we've had a number of companies who have been nesting the studies within a registry and we've already been finding a reduction in cost of forty to sixty percent not to mention expediting the time we get the answers to questions in fact in cases where traditionally we may not even get the answer because patients would enroll in the studies now we're getting answers to questions and we're getting in a timely manner so what does the world look like as we move forward this was the world of a few years ago and we think about premarket review and traditional kind of post market surveillance I mentioned about this pathway for breakthrough devices these more flexible benefit risk policies we put in place and that has really changed the point at which there is market entry and we think rational market entry for technologies and we're starting to see some technologies and companies bringing their devices to the u.s. earlier or first which they were not doing a few years ago but nest is the game changer because with that we have the opportunity to set the needle depending upon the kind of technology in a very different place to allow for more faster market entry in some cases answering those questions or in a timely manner post market and with the kind of safety net we need in place and that becomes a major driver for both device innovation for safer more effective products and for better protection of for patients once that devices on the market and that is the future that we are building towards so let me stop there say thank you and if anyone has any questions I'm happy to take a question or two I think I have two and a half minutes left thank you thank you so much chef this is really exciting a couple questions is nest out now and the unique device ID how does that help nest so nest is not out now the the next step is will we get the basic funding support to get nest off the ground we are in discussions with industry as a part of our user fee program which gets reauthorized next year this is one of the topics of discussion between us and and the device industry and this is out publicly we put out our meeting minutes of those discussions and we've been in discussions with members in Congress whether Congress would provide funding you know I mentioned the Sentinel system for drugs so Congress gave 25 million dollars and the drug industry spends about 20 million dollars a year to support Sentinel and that today is purely for post market surveillance and money just to the FDA here we're talking about handling both sides pre market and post market in a very different model where the money goes to that independent coordinating Center and the stakeholders folks sitting in this audience start having much more of a say in that system so hopefully we'll get that money and if we do then nest will become a reality I think we'll be about 18 months from that time before we have a rudimentary system why because we've already been putting in the building blocks to date so the unique ID then helps to identify what device was used in the patient whereas let's say you've claims data today typically the code isn't sufficiently granular so you don't know which device by which maker and which version was used in the patient same in electronic health records but the UDI identifies who made it the version of it so you know exactly what was used in that patient about sugary I'm the vice chair will be divine I didn't know Fairfax thank you for coming and sharing your time with us very instructive I was wondering with the nest change the 510 K process in terms of approval of new devices or because from my point of view that seemed to be very a lot of errors sneaking to the system where people would come up with devices that say it's analogous to something that existed before we don't need to have to show you the preliminary results to introduce it into the market and then we're stuck with the complications yeah and that's exactly right so under the standards to come to market is truly the high risk the innovative lower risk devices that have clinical trials clinical data to demonstrate the benefits the risks and the benefits outweigh the risks and then you have all these devices that kind of the me toos and they come on by showing that they are technologically similar they're substantially equivalent to another device on the market therefore they piggyback on the idea of the first device is safe and effective and they must be safe and effective what this solves here is getting the better data to actual clinical data on those devices but not because it's something they need to come to market but once on the market that are understanding what they do if there are safety problems we can address them if they're truly better than other technologies out there people should know that and there's an opportunity then to use them or wisely and then feed them back that information back to the developers for them to make better technology I'm very excited by this because when we think of the vaginal mesh complication debacle that we had it took literally five years and what is it like three hundred thousand implants before that was called having the live data to show the complication is really exciting yes I if we had Neff set up and we would have identified those issues with mesh we think you know years earlier in some of the previous sections we've been talking a lot about how electronic health records and devices are working together now for early detection of certain conditions we talked about sepsis a lot and really what we're talking about there it's clinical decision support and so kind of the two-part question there would be I mean how does something like that where you're talking about interaction within the world of devices fit into a model like this and also you know as we're hearing it's being implemented more and more in a lot of the hospitals that we work with kind of how does that how how's that on the roadmap for FDA right now ya know it's it's a great question so yes we're increasingly seeing clinical decision support software you know tools out there well we have not put out a formal policy a traditional regulatory model it's not going to work on clinical decision support you're not going to see those tools come to market as part of the problem because the best ones have more of a learning loop to them you know they're good enough than you learn you're constantly iterating you can't keep coming back in the door and a regulatory system it just won't work so our big what we can we're we care about the most is if the data points that go into such a tool are coming from a more traditional device diagnostic then we want to make sure that information is accurate but then the algorithm that is trying to more replicate how you'd think about the use of that information what it means would be something that we are not likely to actively regulate but it's still we still for this country don't have a solution on how we better understand what it does and we think being able to leverage data that's otherwise collected and to truly get to that that will take time that is the better answer and that becomes both a feedback loop in terms of improving the technology and I think a better safety net on its use thank you very much for the presentation is really interesting um I can see how the the notion of decreasing the time for premarket clearance would help allow products to get to market faster and then potentially deal with dissing issues would you envision nest also encompassing wellness devices though in addition to traditional medical devices so the answer is the answer is yes it's not that you you decide a technology is under it but if you already have data that's being collected on it you leverage it and to the extent there's value there's return on the investment for leveraging it because it's a value to a particular stakeholder group then you'll have the marketplace to do so I don't foresee registries collecting for the truly low-risk but a number of them will be collected in other data sources like electronic health records and they have a way to go before you can make widespread use of them registries today though we are already using because many of them had better curation already built into them that's half the problems with electronic health records now great curation alright thank you very much
2021-03-13 12:45


