Light activated resin | Wikipedia audio article
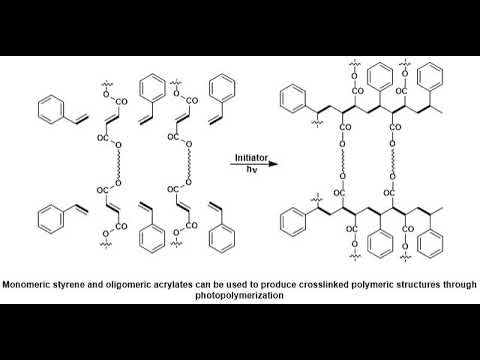
A, photopolymer. Or, light-activated. Resin. Is a polymer. That changes, its properties when. Exposed, to light often, in the ultraviolet. Or visible region. Of, the electromagnetic, spectrum. These. Changes. Are often manifested. Structurally. For example. Hardening. Of the material. Occurs, as a result of, cross-linking. When exposed, to light an. Example. Is shown below, depicting. A mixture, of monomers. Oligomers. And photo. Initiators. That, conform, into, a hardened, polymeric, material. Through a process. Called curing. A. Wide. Variety of, technologically. Useful. Applications. Rely, on photo, polymers. For, example. Some enamels, and varnishes. Depend, on photo polymer. Formulation. For proper, hardening, upon exposure, to light, in. Some, instances. An enamel. Can cure in a fraction, of a second, when exposed, to light as opposed. To thermally. Cured enamels, which, can require a half, an hour or longer. Curable. Materials. Are widely used for, medical, printing. And photoresist, technologies. Changes. In structural. And chemical, properties can. Be induced, internally. By chroma, Falls that the polymer, subunit, already, possesses, or, externally. By addition. Of, photosensitive. Molecules. Typically. A photo polymer, consists. Of a mixture, of, multifunctional. Monomers, and oligomers, in, order, to achieve the desired physical. Properties. And therefore. A wide variety of monomers. And oligomers, have, been developed, that can polymerize, in. The presence, of light either through, internal, or external. Initiation. Photo. Polymers. Undergo. A process. Called curing. Where oligomers, are, cross-linked. Upon exposure, to light for, me what is known as a network, polymer. The. Result, of photo curing. Is the formation, of a thermoset. Network, of polymers. One. Of the advantages. Of photo, curing. Is that it can be done selectively. Using, high-energy, light, sources, for, example lasers. However, most, systems. Are not readily, activated. By light and in, this case a photo, initiator, is. Required. Photo. Initiators. Are compounds. That upon, radiation. Of light decomposed. Into, reactive. Species, that, activate, polymerization, of. Specific. Functional, groups on the oligomers, an. Example. Of a mixture, that undergoes. Cross-linking. When exposed, to light is, shown below. The. Mixture, consists. Of monomeric, styrene. And oligomeric, acrylates. Most, commonly, photo, polymerised, systems. Are typically, cured, through UV, radiation. Since, ultraviolet. Light is more energetic, however, the development, of dye based photo, initiator, systems. Have allowed for the use of visible, light having, potential. Advantages. Of processes. That are more simple, and safe to handle. UV. Curing, in industrial. Processes has, greatly, expanded. Over, the past several, decades. Many. Traditional. Thermally. Cured, and solvent, based technologies. Can be replaced, by, photo, polymerization. Technologies. The. Advantages. Of photo. Polymerization. Over. Thermally, cured, polymerization. Include. High rates of polymerization, and. Environmental. Benefits from. Elimination, of volatile. Organic, solvents. There are two general, routes for photo, initiation. Free, radical. And ionic. The. General, process, involves. Doping, a batch of neat polymer. With small, amounts, of photo initiator. Followed. By selective. Radiation. Of light resulting, a highly, cross-linked. Product. Many. Of these reactions. Do not require, solvent. Which eliminates. Termination. Path via, reaction. Of initiators. With solvent, and impurities. In addition. To decreasing, the overall cost. Topic. Ionic. Mechanism. In, ionic, curing. Processes, an ionic. Photo initiator, is. Used, to activate the functional, group of the oligomers, that, are going to participate in. Cross-linking. Typically. Photo, polymerization. Is, a very, selective. Process. And it is crucial that the polymerization. Takes, place, only where, it is desired, to do so, in. Order to satisfy this. Liquid, NEET oligomers.
Can Be doped with either anionic. Or, cationic photo. Initiators. That will initiate. Polymerization. Only when radiated. With light. Monomers. Or functional. Groups employed. In cationic. Photo, polymerization. Include. Styrene. Ik compounds. Vinyl, ethers, n vinyl. Karbas, all's lactones. Lactams. Cyclic. Ethers, cyclic. Acetal x' and cyclic. Silicones. The. Majority. Of ionic, photo, initiators. Fall, under, the cationic, class, anionic. Photo, initiators. Are considerably. Less, investigated. There. Are several classes of, cationic. Initiators. Including. Own iam salts. Organometallic. Compounds. And pyridinium, salts. As. Mentioned. Earlier one, of the drawbacks of, the photo initiators. Used, for photo, polymerization. Is, that they tend to absorb, in the short UV, region. Photosensitizers. Or chroma, Falls that, absorb, in a much longer, wavelength, region. Can be employed, to excite, the photo initiators. Through an energy, transfer. Other. Modifications. To these types, of systems, are free radical. Assisted. Cationic. Polymerization. In. This case a free, radical, is formed from another species, in, solution that. Reacts with the photo initiator, in, order, to start, polymerization. Although. There are a diverse, group of compounds. Activated. By cationic. Photo, initiators. The, compounds. That find most industrial. Uses contain. Epoque sides, occitane. X' and vinyl. Ethers. One. Of the advantages. To using cationic. Photo. Polymerization. Is, that once the polymerization, has. Begun, it is no longer sensitive. To oxygen, and does not require an, inert atmosphere to. Perform, well. Photolysis. Ah. -. All. Plus. X. Minus. H. V. R. Minus. R. Plus. X. Minus. R. +. +. R. +. X. -. Mhm. R. Plus. Minus. H. Plus. M. Minus. R. Plus. X. Minus. R, plus. H. Plus. X. Minus. Displaced. I'll begin matrix. C. RR. Plus X Charat, 2 H v, RR plus. X Charat. Asked, to our carrot. Plus, plus, our carrot. Plus. X Charat, 2 c. MH. R plus, h plus, mr. Plus X Charat, 2 R plus, h plus X carrot, end matrix. M. Equals. Monomer. Equals. Topic. Cationic. Photo. Initiators. Equals. The. Proposed. Mechanism. For cationic, photo. Polymerization. Begins. With the photo excitation, of. The initiator. Once. Excited. Both homolytic. Cleavage and, dissociation. Of a counter, anion, takes place. Generating. Cationic. Radical. Are an, aryl, radical. Are and, unaltered. Counter, anion, X. The. Abstraction. Of a Lewis acid in figure, above a hydrogen. By the cationic. Radical. Produces. A very, weakly, bound hydrogen. And a free radical. The. Acid, is further. Deprotonated. By the anion. X in, solution. Generating. A Lewis acid with, the starting, an iron X as, a counter. Ion, it. Is thought that the acidic, proton, generated. Is what ultimately, initiates. The polymerization. Equals. Topic. Owned iam salts. Equals. Since. Their discovery in. The, 1970s. Erol own IAM salts, more specifically. I adonia, manned sulfonium, salts. Have received, much attention, and, have found many industrial. Applications. Other. Less-common. Own, IAM salts, not mentioned, here include, ammonium. And phosphonium, salts. The. Typical, own IAM compound. Used, as a photo, initiator. Contains. Two all three, RNA, groups, for i adonia, man sulfonium. Respectively. Own. IAM salts, generally, absorb, short, wavelength. Flight in the UV, region spanning. From. 225. To, 300. Nanometers. One. Characteristic. That is crucial, to the performance. Of the own IAM photo, initiators. Is that the counter, anion, is non-nucleophilic. Since. The bronsted, acid, generated. During the initiation step. Is considered. The active, initiator. For polymerization. There. Is a termination. Route, where, the counter, ion, of the acid, could act as the nucleophile. Instead. Of a functional, groups, on the oligomers. Common. Counter, anions, include, bf, minus, 4p. F minus, 6a, SF. Minus, six SB f, minus, six. There. Is an indirect. Relationship. Between the, size of, the counter, ion, and percent, conversion. Topic. Organometallic. Although. Less common. Transition. Metal, complexes, can. Act as cationic. Photo, initiators. As well, in. General, the mechanism. Is more simplistic, than, the own IAM ions, previously. Described. Most. Photo, initiators. Of this class consist. Of a metal salt, with a non, nucleophilic. Counter, anion. For. Example. Ferrous, inium salts, have received, much attention for. Commercial, applications. The. Absorption, band, for, ferrous, inium salt derivatives.
Are In a much longer, and sometimes. Visible, region. Upon. Radiation. The metal center, loses, the ligands, and the ligands, is replaced, by functional. Groups that begin, the polymerization. One. Of the drawbacks of, this method, is a greater, sensitivity, to. Oxygen. There. Are also several. Organometallic. Anionic, photo, initiators. Which, react, through, a similar, mechanism. For. The anionic, case, excitation. Of a metal centre is followed, by either heterolytic. Bond, cleavage, or, electron. Transfer. Generating. The active, anionic, initiator. Topic. Pyridinium. Salts. Generally. Pyridinium, photo. Initiators. RN substituted. Pyridine, derivatives. With a positive, charge placed, on the nitrogen. The. Counter, ion, is in most cases a. Non, nucleophilic. Anion, upon. Radiation. Homolytic. Bond cleavage, takes, place, generating. A pyridinium, cation. Achra, Dickel and a neutral, free radical. In. Most, cases, a, hydrogen. Atom is abstracted. From the oligomers. Pyridinium. Radical. The. Free radical. Generated. From the hydrogen, abstraction is. Then terminated. By the free, radical. In solution. This. Results. In a strong, pyridinium, acid. That can initiate. Polymerization. Topic. Free. Radical. Mechanism. Nowadays. Most, radical. Photo, polymerization. Pathways. Are based on addition, reactions. Of carbon, double, bonds, in acrylates, or metric, relates, and these, pathways are widely, employed, in photo lithography in. Stereo, lithography. Before. The free radical, nature of certain. Polymerizations. Was determined. Certain. Monomers, were observed, to polymerize, when, exposed, to light, the. First to demonstrate, the photo induced. Free, radical. Chain reaction. Of vinyl, bromide was. Ivan Ostrom is Lenski, a russian chemist who, also studied. The polymerization. Of, synthetic rubber. Subsequently. Many, compounds. Were found to, become, dissociated. By light and found immediate. Use as photo, initiators. In the polymerization. Industry. In. The free radical, mechanism of. Radiation. Curable, systems, light absorbed. By a photo, initiator. Generates. Free, radicals. Which, induce, cross-linking. Reactions. Of a mixture of functionalized. Oligomers. And monomers. To generate, the cured film. Photo. Curable. Materials. That form through, the free radical, mechanism, undergo. Chain, growth polymerization. Which. Includes, three basic, steps, initiation. Chain. Propagation. And chain, termination. The. Three steps, are depicted. In the scheme below, where R represents the. Radical, that forms, upon interaction. With radiation, during, initiation and, M is a monomer. The. Active, monomer, that is formed, is then propagated. To create growing, polymeric, chain, radicals. In. Photo, curable. Materials. The propagation, step, involves. Reactions. Of the chain radicals. With, reactive. Double, bonds, of the prepolymer, Zoar oligomers. The, termination. Reaction. Usually, precedes, through, combination. In which to chain radicals. Are joined together all, through. Disproportionation. Which, occurs, when an, atom, typically. Hydrogen. Is transferred. From one radical chain, to another resulting. In two polymeric, chains. Initiation. Are carat, bullets, see our, carrot. Bullet + M + C, to, room, carat bullet end aligned. Greater, than i ni, t IA, t io, r, + h nu, r r+. M, room, display. Style, begin, aligned, initiate. A or + H underscore. New, and. Ce2, our carat, bullet, see our, carrot. Bullet + M + c2, room, carrot bullet, end aligned. Propagation. Room + MN, room n + 1 display. Style, C room. Carrot, bullet + M underscore. Math hit enter, room underscore. Math, it n +, 1 carrot, bullet, termination. Combinati. On. Room. N. Plus. M. M. Ah. Reum. N. M. M. Our. Display. Style, C room, underscore. Method, and carat, bullet, plus carat. Bullet M underscore. Moffett. Mr2. Room underscore. Moffett. N m underscore. Moffett, M R. Disproportionation. Room. N. Plus. M. M. Our. Room. N. Plus. M. M. Our. Display. Style, C, room. Underscore. Method, and carat, bullet, plus carat. Bullet M underscore. Moffett. Mr2. Room, underscore. Method, n plus, M underscore. Moffett, M are. Most. Composites. That cure, through, radical chain, growth, contain, a diverse. Mixture, of oligomers, and monomers. With functionality. That, can range from two, to eight and, molecular weights, from 500. 3000. In. General. Monomers. With higher, functionality. Result, is a tighter, cross-linking. Density. Of the finished, material. Typically. These oligomers, and monomers. Alone, do not absorb, sufficient. Energy for. The commercial. Light sources, used. Therefore, photo. Initiators. Are included. Topic. Photo. Initiators. There. Are two types of free radical. Photo annotators. A two-component. System. Where. The radical is, generated. Through abstraction. Of a hydrogen atom. From, a donor compound. Also called, Co initiator. And the one component, system. Where two radicals. Are generated, by cleavage. Examples. Of each type of free radical. Photo initiator, is. Shown, below. Bend's, often on zan phones, and Quinn, owns are examples. Of abstraction. Type photo, initiators. With, common, donor compounds.
Being Aliphatic. Amines. The. Resulting. Are species. From the donor compound. Becomes, the initiator. For the free radical. Polymerization. Process. While, the radical, resulting. From the starting, photo initiator, bends. Often on in the example. Shown above is typically. Unreactive. Benzoin. Ethers, acetophenone. X' benzyl. Ox amazed and acyl. Phosphenes, are some examples. Of cleavage, type photo, initiators. Cleavage. Readily, occurs, for, the species to, give two radicals. Upon, absorption. Of light and, both radicals. Generated can. Typically, initiate. Polymerization. Cleavage. Type, photo, initiators. Do, not require, a CO initiator. Such, as aliphatic. Amines. This. Can be beneficial, since, amines, are also effective. Chain transfer, species. Chain. Transfer, processes. Reduce, the chain length and ultimately, the, cross link density of. The resulting. Film. Topic. Oligomers. And monomers. The, properties, of a photo cured, material such. As flexibility. Adhesion. And chemical. Resistance. Are provided. By the functionalized. Oligomers, present. In the photo curable. Composite. Oligomers. Are, typically, Epoque sides. Urethanes. Polyethers. Or, polyesters. Each of which provides, specific. Properties, to, the resulting, material, each. Of these oligomers, are, typically, functionalized. By, an acrylate, an, example. Shown below is an epoxy oligomers. That, has been functionalized. By, acrylic, acid, ik, related. Epoxies. Are useful. As coatings, on metallic, substrates. And result. In glossy, hard coatings. Ik. Related. Urethane. Oligomers, are, typically, abrasion. Resistant. Tough, and flexible. Making ideal. Coatings. For floors, paper. Printing. Plates and packaging. Materials. Acylated. Polyethers. And polyesters. Result, in very hard solvent. Resistant. Films, however. Polyethers. Are prone to UV. Degradation and. Therefore, are rarely used in UV, curable, material. Often. Formulations. Are composed, of several types of oligomers, to, achieve the desirable, properties, for. A material. The. Monomers, used, in radiation, curable. Systems. Help, control, the speed of cure cross, link density. Final. Surface properties. Of the film and viscosity, of the resin. Examples. Of monomers, include, styrene. And vinyl. Pyro lead own and acrylates. Styrene. Is a low cost monomer. And provides, a fast cure, n vinyl. Pyro lead own results, in a material. That is highly, flexible when. Cured, has low, toxicity, and, acrylates, are highly, reactive, allowing. For rapid, cure rates and are highly, versatile. With monomer, functionality. Ranging. From mana functional, to tetra functional. Like. Oligomers. Several. Types of monomers, can be employed, to achieve, the desirable, properties of, the final material. Topic. Applications. Photo. Polymerization. Is, a widely, used, technology. Used, in applications. Ranging, from, imaging, to biomedical, uses. Below. Is a description. Of just some photo, polymerization. Applications. Topic. Dentistry. Dentistry. Is one market, where, free-radical, photo polymers. Have found wide usage as, adhesives. Sealants. And. Protective. Coatings. These. Dental. Composites, are, based on a camp, or quinone, photo initiator, and, a matrix, containing. Metric, relate oligomers, with, inorganic. Fillers such, as silicon. Dioxide. Resin. Cements. Are utilized, in, looting, cast ceramic. Full porcelain. And veneer. Restorations. That are thin or translucent. To permit visible, light penetration. And thus polymerized. The cement. Light-activated. Cements. May be, radiolucent. And are usually, provided. In various, shades since, they are utilized.
In, Aesthetically. Demanding. Situations. Conventional. Halogen. Bulbs, Argan, lasers, and xenon arc lights are currently, used, in clinical, practice a. New. Technological, approach. For, curing, light activated. Oral. Biomaterials. Is presented. The. New light curing, unit, LCU. Is based, on blue light-emitting, diodes. LED. The. Main benefits. Of LED, LCU, technology. Are long, lifetime. Of LED, LCU. Several. Thousand, hours no, filters, or cooling, fan required. Virtually. No decrease, of light output. Over lifetime with. Resulting. Consistent. And high quality of. Material. Curing. Simple. Depth of cure experiments. Of dental, composites, cured, with LED, technology, show. Promising. Results. Topic. Medical. Uses. Photo. Curable. Adhesives. Are also used in the production of, catheters. Hearing. Aids, surgical. Masks, medical. Filters, and blood, analysis. Senses. Photo. Polymers. Have also been, explored. For uses, in drug delivery tissue. Engineering. And cell encapsulation. Systems. Photo. Polymerization. Processes. For, these applications, are. Being developed, to, be carried, out in vivo, or ex-vivo. In. Vivo, photo, polymerization. Would, provide, the advantages. Of production. And implantation. With, minimal, invasive, surgery. Ex vivo, photo. Polymerization. Would, allow for fabrication. Of complex, matrices, and versatility. Of, formulation. Although. Photo polymers. Show promise for, a wide range of, new biomedical. Applications. Biocompatibility. With. Photo polymeric. Materials. Must still be addressed. And developed. Topic. 3d. Printing. Stereo. Lithography. Digital. Imaging, and 3d. Inkjet, printing, are just a few 3d. Printing, technologies. That make use of photo, polymerization. Pathways. 3d. Printing, usually, proceeds. With CAD cam software which. Creates, a 3d, computer model. To be translated. Into a 3d, plastic, object. The. Image is, cut in slices where. Each slice, is, reconstructed. Through radiation, curing. Of the liquid, polymer, converting. The image into a solid, object. Photo. Polymers. Used, in 3d imaging. Processes. Requires. Sufficient. Cross-linking. And should ideally, be designed, to have a minimal, volume, shrinkage, upon, polymerization. In, order, to avoid distortion. Of the solid, object. Common. Monomers, utilized. For, 3d, imaging, include. Multifunctional. Acrylates and metric, relates, often. Combined, with a non polymeric. Component. In order to reduce volume shrinkage. A. Competing. Composite. Mixture, of epoxy, resins, with cationic. Photo, initiators. Is becoming. Increasingly used. Since, their volume, shrinkage. Upon, ring-opening polymerization. Is. Significantly. Below those, of AK relates and metric, relates. Free. Radical. And cationic. Polymerization. X', composed, of both Epoque site and acrylate, monomers. Have also been employed, gaining. The high rate of polymerization. From. The acrylic. Monomer, and better, mechanical. Properties, from, the epoxy. Matrix. Topic. Photoresists. Photoresist. Sir coatings, or oligomers. That, are deposited on a surface. And they're designed to change properties. Upon, a radiation. Of light. These. Changes. Either polymerize. The liquid, oligomers, into, insoluble. Cross-linked. Network polymers. Or decomposed, the. Already, solid, polymers. Into liquid, products. Polymers. That form, networks during. Photo. Polymerization. Are, referred, to as, negative, resist. Conversely. Polymers. That decompose. During, photo, polymerization. Are, referred, to as, positive. Resists. Both. Positive. And negative resists. Have found many, applications. Including, the design, and production of. Microfabricated. Chips. The. Ability, to patent, the resist, using, a focused, light source, has, driven the field of photo lithography. Topic. Negative. Resists. As. Mentioned. Negative. Resists. Or photo polymers. That become, insoluble, upon. Exposure, to radiation. They. Have found a variety of, commercial. Applications. Especially. In the area of designing. And printing small. Chips, for electronics. A. Characteristic. Found, in most negative. Tone resists. Is the presence, of, multifunctional. Branches. On the polymers. Used. Radiation. Of the polymers, in the presence, of an in XI ater results. In the formation, of, chemically. Resistant. Network polymer, a, common. Functional. Group used, in negative, resist, is epoxy. Functional. Groups. An. Example. Of a widely, used polymer. Of this class is, su 8. Su. 8 was, one of the first polymers.
Used, In this field and found, applications. In wire board, printing. In. The presence, of a cationic. Photo, initiator, photo. Polymer su. 8 forms, networks, with, other polymers, in solution. Basic. Schemes shown below. Su-8. Is an example, of, an intramolecular. Photo, polymerization. Forming. A matrix, of cross-linked. Material. Negative. Resists. Can also be, made using Co. Polymerization. In. The event that you have two, different monomers or oligomers. In, solution. With multiple. Functionalities. It, is possible. For the two to polymerize, and, form a less soluble, polymer. Manufacturers. Also use, light, curing, systems. In OEM, assembly. Applications. Such, as specialty. Electronics. Or medical. Device, applications. Topic. Positive. Resists. As. Mentioned. Positive. Resist, exposure, to, radiation, changes. The chemical structure. Such that it becomes a liquid or, more soluble. These. Changes. In chemical structure, are, often, rooted in the cleavage, of specific. Linkers, in the polymer. Once. Irradiated. The, decomposed. Polymers. Can be washed away using, a developer. Solvent, leaving, behind the polymer, that was not exposed, to light. This. Type of technology. Allows. The production of very fine stencils. For applications. Such as micro. Electronics. In. Order, to have these types, of qualities, positive. Resists, utilize, polymers. With labile, linkers, in the backbone, that can be cleaved upon, a radiation. Or using, a photo generated. Acid. To hydrolyze, bonds. In the polymer, a, polymer. That decomposes. Upon, a radiation. To a liquid or more, soluble, product. Is referred, to as a positive tone, resist. Common. Functional. Groups, that can be hydrolyzed. By photo, generated. Acid, catalysts, include, poly carbonates. And polyesters. Topic. Fine, printing. Photopolymer. Can, be used to generate printing. Plates which, are then pressed onto, paper like metal, type, this. Is often used in modern, fine printing. To achieve the effect of embossing, or the, more subtly. Three-dimensional. Effect of letterpress, printing, from, designs, created. On a computer. Without needing, to engrave, designs, into, metal, or cast metal, type, it. Is often used, for business, cards. Topic. Repairing. Leaks. Industrial. Facilities. Are, utilizing. Light activated, resin. As a sealant, for leaks and cracks. Some. Light activated. Resins. Have unique, properties, that make it ideal as, a pipe, repair, product. These. Resins. Cure, rapidly. On any wet or dry surface. Soppec. Fishing. Light-activated. Resins. Recently. Gained a foothold with, fly, tiers as a way to create, custom, flies in, a short period of time with. Very little cleanup, involved. Soppec. Floor. Refinishing. Recently. Light-activated. Resins. Have found a place in floor, refinishing. Applications. Offering. Instant, return, to service not, available. With any other chemistry. Due, to the need to cure, at ambient, temperatures. The. Cause of application. Constraints. These coatings. Are exclusively. UV, cured, with portable, equipment, containing. High-intensity. Discharge, lamps. Such. UV, coatings. Are now commercially. Available for. A variety of substrates. Such. As wood vinyl, composition tile. And concrete, replacing. Traditional poly, urethanes. For, wood refinishing. And low durability, acrylics. For VCT. Topic. Environment. Pollution. While, washing, the polymer, plates after they have been exposed, with, ultraviolet. Light with water and brushes, monomers. Will enter the sewer system. Eventually, adding. To the plastic, content, of the oceans. Current. Water, purification. Installations. Are not able to remove monomer. Molecules. From sewer water. Some. Monomers, such, as styrene. A toxic. Or carcinogenic.
2019-06-09 03:57